Improved adhesion of coating on heat-resistant materials ――Role of doping elements revealed for the first time――
- Headline
- Press Release
Highlights
◆The role of alloying elements on the adhesion of coatings on heat-resistant materials was clarified.
◆It was clarified that elements that significantly lower the interfacial energy improve the adhesion.
◆These results are expected to be effective in preventing spalling and extending the service life of Thermal barrier coatings.
Abstract
A research group of the University of Tokyo (UTokyo), Graduate School Frontier Science (GSFS), NIMS, and Kawasaki Heavy Industries, Ltd., has clarified the mechanism of adhesion enhancement between a bond coat (Note 2) and an oxide (TGO, Note 3) formed on a bond coat in a Ni-based superalloy (Note 1) by first-principles calculations (Note 4). The group members are Masahiro Negami (PhD student in UTokyo, GSFS, and Kawasaki Heavy Industries, Ltd.), Tessei Yoshino (Master student at the time of research), Yoko Yamabe-Mitarai(Professor), Ryoji Sahara (Group Leader, NIMS), and Ryo Morihashi (Kawasaki Heavy Industries, Ltd.,).
Systematic calculations of the interfacial energy between the bond coat and oxide, when Group 3 (Note 5) and Group 4 elements are added, have revealed for the first time. It was found that Group 4 elements lower the interfacial energy more effectively than Group 3 elements and that Group 14 elements, Si, lower the interfacial energy the most. The element that lowers the interfacial energy was found to improve adhesion.
This is the first clarification of the mechanism of adhesion improvement, and is expected to contribute to extending the service life of coating materials (Note 6).
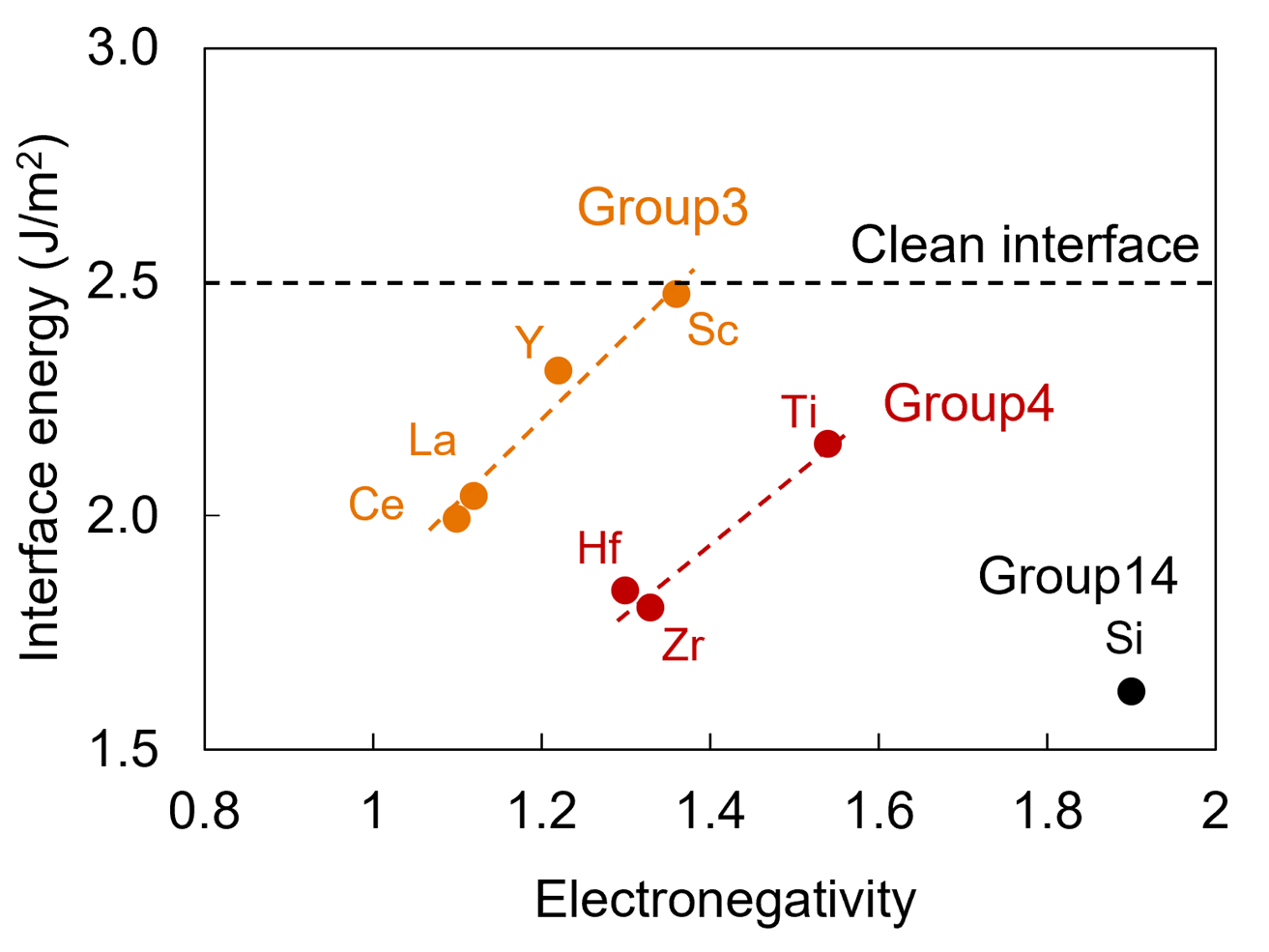
Figure 1: Interfacial energy change by doping of elements
The interfacial energy by doping of an element decreases in the order of Si, Group 4 elements, and Group 3 elements. The smaller the electronegativity, the lower the interfacial energy among elements of the same group.
Contents
Ni-based superalloys are used in turbines in jet engines operated at the highest temperature. Since they are used under combustion gases higher than the melting point, thermal barrier coatings are required. The thermal barrier coating consists of multiple layers of bond coat and top coat, but during long operation at high temperatures, oxides (TGO, Note 3) are formed between the bond coat and top coat (Note 6). Cracks between the bond coat and oxide cause spalling of the coating material, so Hf or Si is doped to improve adhesion. Although it was known that this can extend the life of the coating material, the reason for this was not clear.
In order to clarify the effect of the alloying elements on the interface between the bond coat and the oxide, we calculated the interfacial energy using first-principles calculations. The interface energy was calculated by superimposing the (111) surface of Ni, a representative element of the bond coat (NiCoCrAl), and the (0001) surface of Al2O3, a TGO, as shown in Figure 2, and doping elements at the interface.
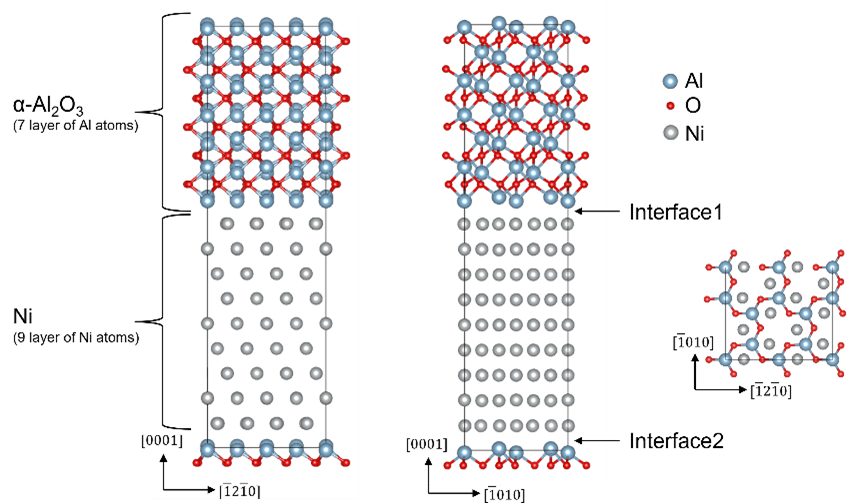
Figure 2 Interface model of Ni and Al2O3
As shown in Figure 1, Group 4 elements were found to lower the interfacial energy more than Group 3 elements, and Si further lowers the interfacial energy. In addition, among elements of the same group, elements with smaller electronegativity were found to lower the interfacial energy. The charge density difference (Note 7) shown in Figure 3 indicates that the Group 4 elements Hf, Zr and Si bond strongly (red color) to Ni. It reveals for the first time that the elements that lower (stabilize) the interfacial energy bond strongly to Ni, thereby improving adhesion. As shown in the results of the cyclic oxidation test in Figure 4, the number of cycles to delamination is higher with the simultaneous addition of Y, Hf, and Si than with the addition of Y alone, which can be explained by the calculated results that the addition of Hf and Si strongly bond to Ni.
Until now, doping elements has been selected empirically, but now it is possible to select doping elements to reduce the interfacial energy by calculating in advance. The results of this research are expected to contribute to extending the service life of coating materials by clarifying design guidelines for expanding life of coating materials.
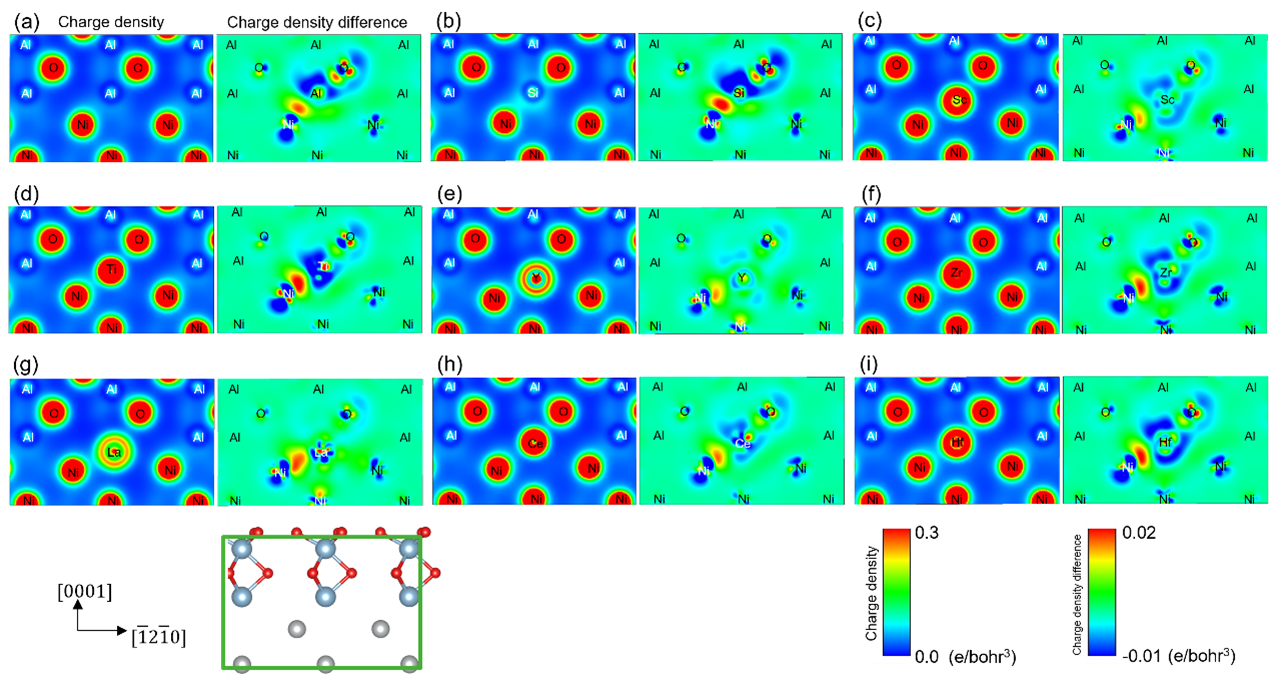
Figure 3: Charge density and charge density difference by doping elements
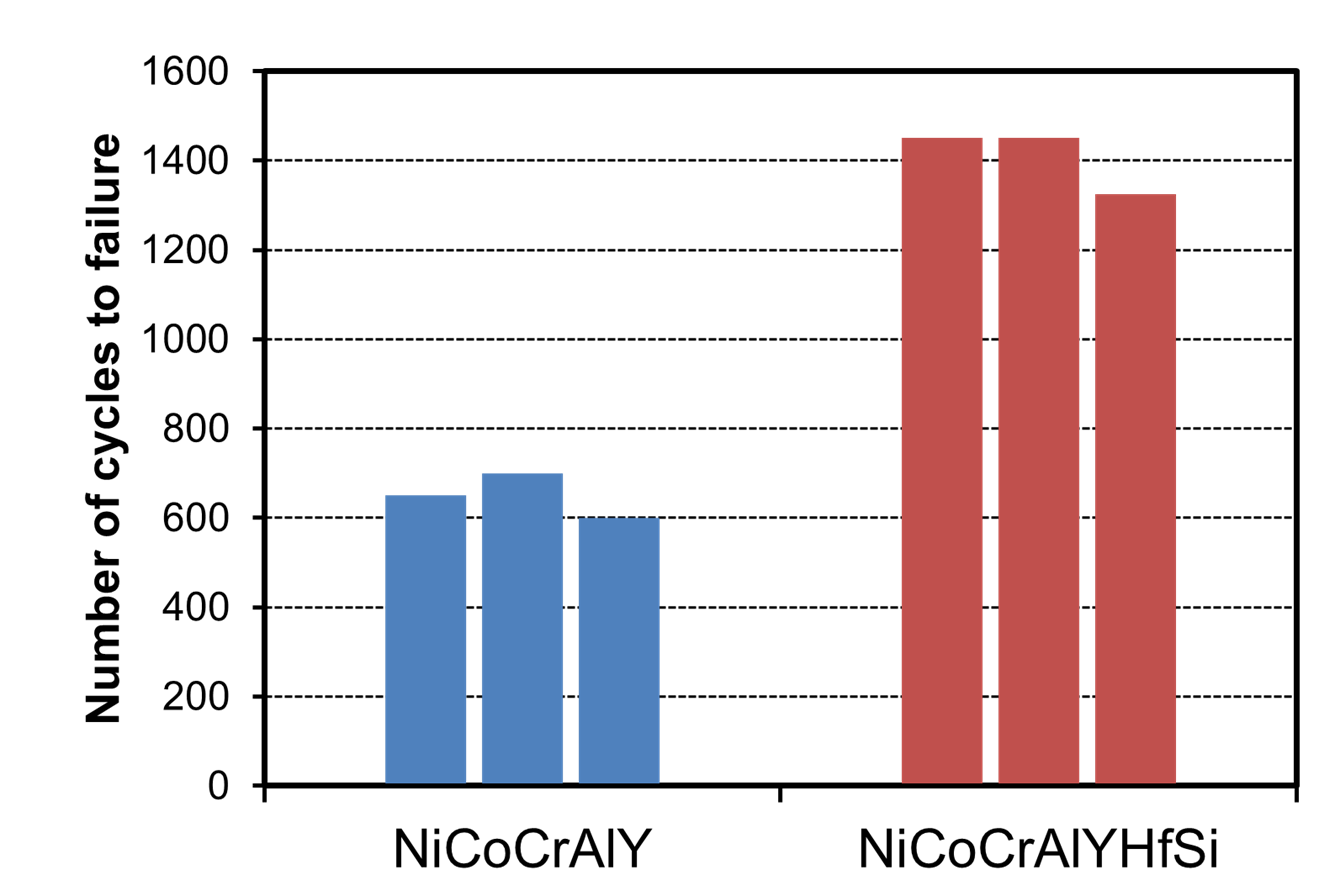
Figure 4 Comparison of the doping effect of Y and simultaneous addition of Y, Hf, and Si on cyclic oxidation test of NiCoCrAl.
(Note 1) Ni based superalloys:
Heat resistant materials used in turbine in jet engine.
(Note 2). Bond coat:
MCrAlY (M: Ni, Co, etc.) is often used to coat the surface of Ni-based superalloys.
(Note 3) TGO:
Thermally Grown Oxide, Oxides, mainly Al2O3 (alumina), are formed between the bond coat and the top coat.
(Note 4) first-principles calculations:
Computational methods based on quantum mechanics that do not use experimental results or empirical parameters
(Note 5) group:
The properties of elements change periodically, and the periodic table arranges atoms in order of atomic number so that elements with similar properties are arranged vertically. A vertical row of elements with similar properties is called a group.
(Note 6) coating:
A coating applied to a Ni-based superalloy to improve its heat resistance; a bond coat is applied to the Ni-based superalloy, and a ceramic such as zirconia is applied as a top coat on surface of the bond coat.
(Note 7) The charge density difference:
Difference between charge density without and with doping elements
Article
Publication: Corrosion Science
Title: Effect of reactive elements in MCrAlX bond coat for durability improvement of thermal barrier coatings
Authors: Masahiro Negami1,3, Ryo Morihashi1,3, Tessei Yoshino1, Ryoji Sahara2, Yoko Yamabe-Mitarai*1
- Department of Advanced Materials Science, Graduate School of Frontier Sciences, The University of Tokyo
- National Institute for Materials Science
- Kawasaki Heavy Industries, Ltd.,
DOI: 10.1016/j.corsci.2024.112329