Frontier Sciences: Makoto Yamagishi

Because various types of cancers and infections are spreading worldwide, the significance of basic research that helps us understand the host and pathogen of diseases has been recognized. Herein, we attempt to elucidate the onset mechanism of different viral infections and intractable blood cancers and develop novel therapeutics and diagnosis methods by integrating various academic fields, including molecular biology, genomics, and data science, and studying genomes and epigenomes.
Makoto Yamagishi
Associate Professor
Laboratory of Viral Oncology and Genomics,
Department of Computational Biology and Medical Sciences
https://square.umin.ac.jp/yamagishi/eng/index.html
I pursued my doctoral studies in molecular biology and basic medical sciences at the Department of Computational Biology and Medical Sciences (formerly known as the Department of Medical Genome Sciences) at the Graduate School of Frontier Sciences, University of Tokyo. My research interests lie primarily in the field of intractable diseases, such as cancer and infections, and the exploration of the underlying natural phenomenon and laws. Specifically, I am now involved in the study of retrovirus infections and hematological malignancies in collaboration with the Life Science Data Research Center (LiSDaC), the Institute of Medical Science, University of Tokyo, and the Pandemic Preparedness, Infection, and Advanced Research Center (UTOPIA), University of Tokyo.
Some pandemic viral infections cause severe cancers after chronic latent infection. Viral tumorigenesis by human T-cell leukemia virus type 1 (HTLV-1)—a retrovirus that infects humans—and Epstein–Barr virus have a long history with humans, and they have been menacing our lives across the globe. Viruses are skilled at affecting host health, causing cancer or chronic inflammation, and are difficult to remove from the human body once incubated. As a result, we are still facing many challenges.
We collaborate with medical institutions all across Japan to collect high-quality clinical samples and data using cutting-edge analytic technologies, such as next-generation sequencing, or NGS, and single-cell analysis, to elucidate clonal evolutions and decode epigenome codes of cancer and infections using experimental biology and data science approaches (Figures 1 and 2). We have identified the mutual interactions between viruses and hosts, various infected cells, and tumor cells, the mechanism of multistep carcinogenesis caused by genomic abnormalities, and new genes responsible for diseases.
 Figure 1
Figure 1
Mechanisms of disease appearance, drug actions, and resistance, and new therapeutic targets can be elucidated by collecting clinical samples in various clinical and medication situations and analyzing the genome, epigenome (chromatin structure, histone modification, methylated DNA, etc.), and gene expression synthetically with clinical data.
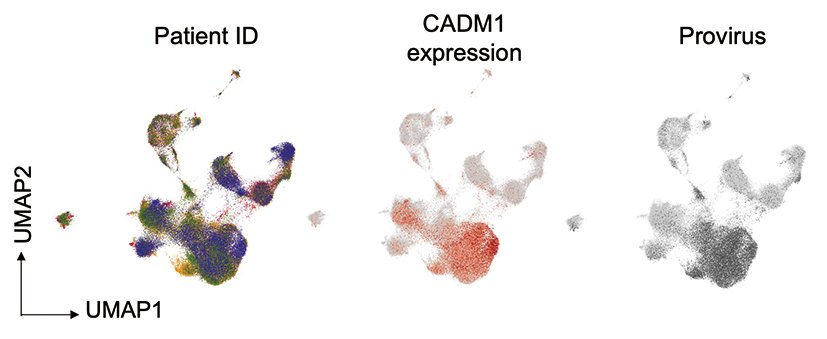 Figure 2
Figure 2
An example of single-cell analysis: Cells are clustered using an algorithm based on the characteristics of each cell and genetic information and classified by color. The development of technology and data science has enabled highly accurate analysis of cells that are infected with a virus or turned into tumors.
“Epigenetic regulation” is the major focus of our study. An epigenome is the control system for genetic information that switches gene expression on and off without alternating DNA sequences. Recently, through big data analysis of epigenomes from clinical samples and experimental verifications, we clarified the agglutination mechanism of DNA compounds (chromatin structure), which occurs in cells infected by malignant lymphoma and HTLV-1, and identified their causes—the EZH1 and EZH2 molecules (Figure 3).
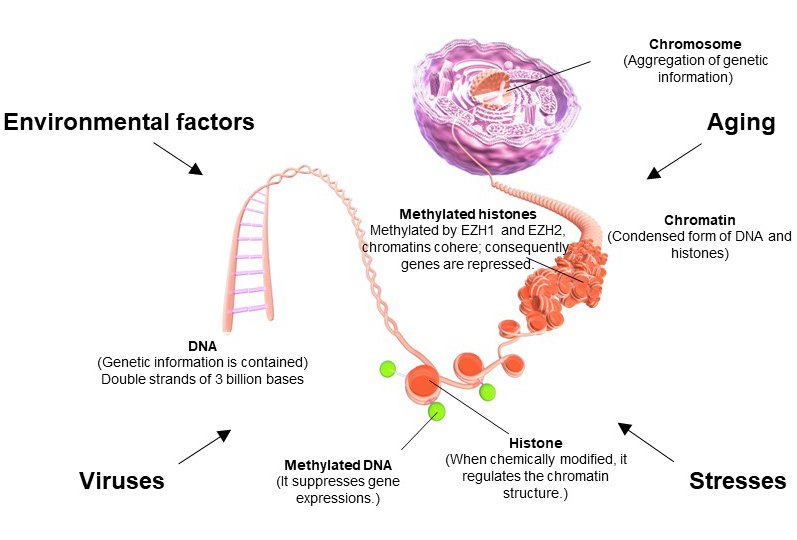 Figure 3
Figure 3
DNA is wrapped around histones and stored in the nucleus in a highly structured form. The use of genetic information (amount and timing) is regulated by an epigenome. Abnormalities in an epigenome are critical because they deviate from genetic information regulation.
There are numerous obstacles that we must overcome on the path from basic research to the development of new drugs. Allegedly, only a few pharmaceuticals and technologies can be realized and used at clinical sites. However, we had opportunities to have industry–academia collaboration associations with pharmaceutical companies and succeeded in developing an inhibitor for EZH1/2, a first-in-class medication (innovative novel drugs), with the help of many researchers. This novel drug developed in Japan was approved in 2022 for treating patients with adult T-cell leukemia (ATL) with an extremely poor prognosis caused by HTLV-1. It has been used for many patients with ATL. Because this drug was added to a limited list of epigenetic therapeutics as a new option, it is expected to be applied to many other diseases.
Having experienced the process starting from identifying molecules responsible for diseases to drug development with an industry–academia collaboration, I was convinced that basic research and an interdisciplinary approach will produce new outcomes with great value. Standing at the intersections of “experimental biology and data science,” “basic research and clinical research,” “infectology and oncology,” and “genome and epigenome,” I am keen on fostering innovations in independent sciences through new ideas and technologies.

vol.43
- Cover
- Energy Technology for Carbon Neutrality
- Mehr Licht!: Realizing Highly Efficient Light Energy Conversion Using Organic Materials
- Decoding Life Phenomena and Intractable Diseases Through Experimental Biology and Data Science
- For a Better Interaction Between Humans and Robots
- GSFS FRONTRUNNERS: Interview with an entrepreneur
- Voices from International Students
- ON CAMPUS x OFF CAMPUS
- EVENT & TOPICS
- INFORMATION
- Relay Essay
