Frontier Sciences: Yasunari Tamai

“Mehr Licht! (More light!)” is allegedly the phrase that the famous German writer Goethe uttered on his deathbed. It sounds like it has a deep meaning; however, he should have simply meant that he wanted more light in the room because it was too dark. While pondering what to write for these Frontier Sciences pages, I noticed that this phrase encapsulates my research. Therefore, I decided to use it as the title of my article.
Yasunari Tamai
Associate Professor,
New Materials and Interfaces
Department of Advanced Materials Science
https://www.organicel.k.u-tokyo.ac.jp/en/
One of the biggest challenges that humans face in the 21st century is the energy problem. Global energy consumption is increasing yearly; however, fossil fuels, including petroleum, coal, and natural gas, are limited. For a country such as Japan, which has low self-efficiency of these fuels, the depletion of fossil fuels is a menacing problem. Nevertheless, solar energy is free for anyone and perpetual (or at least as long as humans exist, so it can be the perfect solution to solve this problem.
I research solar energy conversion using organic materials. One of my primary research themes is the development of organic photovoltaics, which involves the combination of p- and n-type organic semiconductors (Figure 1). When an organic semiconductor absorbs light, it creates an exciton, a bound state of an electron and hole by the Coulomb force. When excitons separate into charges at the interface of p- and n-type organic semiconductors, charges gather at the electrode and promote photoelectric conversion (Figure 2).
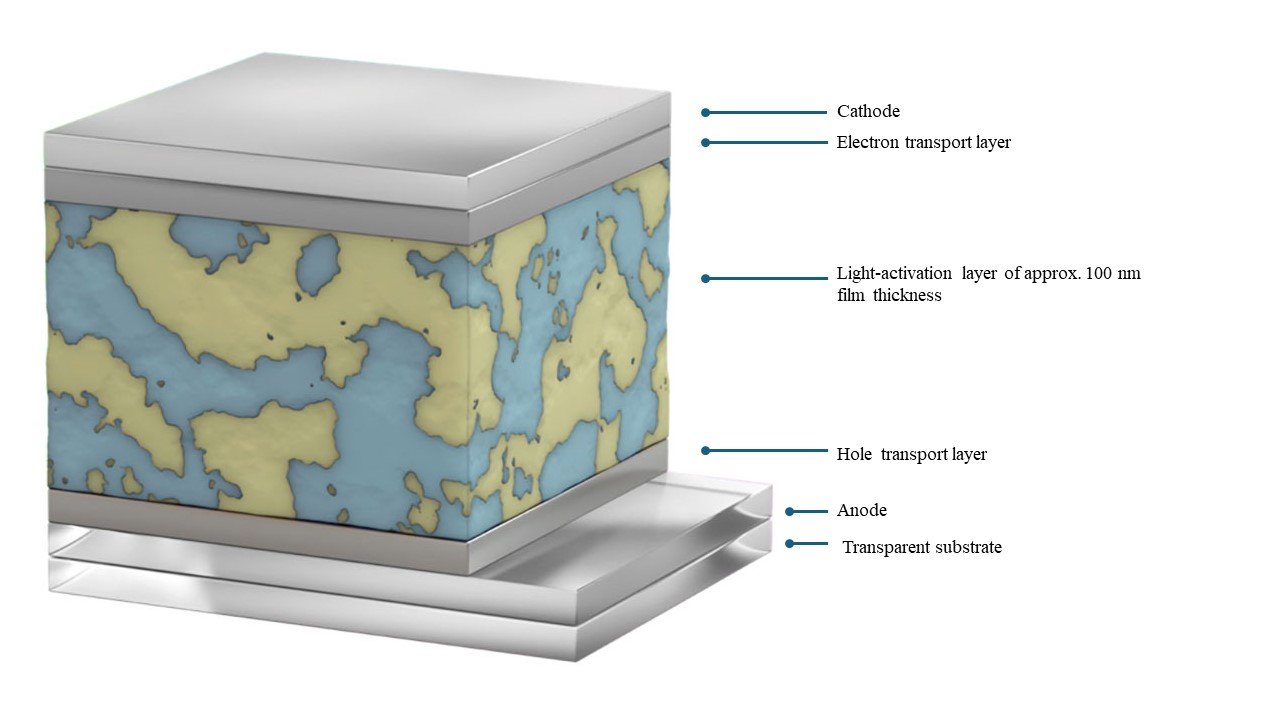 Figure 1
Figure 1
Organic photovoltaics can generate electricity with a photoactivelayer of approximately 100 nm (1/100 of plastic wrap for food)
 Figure 2
Figure 2
Photoelectric conversion of organic photovoltaics: Excitons separate into charges at the phase separation interface and produce free charges.
An organic solar cell is lightweight and flexible and can be mass-produced by printing. It is considered to be the next-generation solar cell (Figure 3); however, the energy loss due to its nonradiative charge recombination is large compared with inorganic materials, such as silicon, which is a challenging issue to address.
 Figure 3
Figure 3
Organic semiconductors can be dissolved into organic solvents. Therefore, a film can be made by printing technique.
 At present, a metal electrode is made by vacuum deposition. We are attempting to make it by printing.
At present, a metal electrode is made by vacuum deposition. We are attempting to make it by printing.
We have analyzed the dynamics of charge recombination and successfully demonstrated that radiative recombination can be accelerated by making the energy level of charge transfer state (an intermediate state of charge separation) close to the energy level of excitons. This can result in organic photovoltaics that emit brighter light with less energy loss.
Another research project I am working on is upconversion emission, which involves the use of organic dye materials. Upconversion emission is fascinating because it allows light emission at a shorter wavelength than light irradiated on a sample (Figure 4). In particular, when using the “triplet-triplet annihilation reaction,*” even weak light energy, such as sunlight, can trigger upconversion emission, making it possible to convert large amounts of near-infrared (NIR) light found in sunlight into high-energy ultraviolet (UV) light.
 Figure 4
Figure 4
Upconversion emission by organic dye materials: Green light sources are upconverted into shorter wavelength blue light.
For example, titanium oxide performs a photocatalytic function known as the Honda–Fujishima effect by absorbing UV light, indicating that most of the sunlight energy is not used. However, NIR radiation can enable titanium oxide to perform catalytic functions by combining photocatalytic materials and upconversion light-emitting devices. We demonstrated that the confinement of triplet excitons in local spaces accelerates exciton annihilation reactions and enhances the efficiency of upconversion emission.
Absorbing and emitting more light are the keys to increasing efficiency in the above research areas. Thus, we will continuously strive to obtain “Mehr Licht!”
NOTE
*Triplet–Triplet Annihilation Reactions:
When two electrons in the frontier orbital spin antiparallel and parallel, they are called singlet and triplet states, respectively. When a triplet exciton encounters another exciton, they form a triplet pair, which is converted into a radiative singlet exciton because the overall spin quantum number of a part of it is zero. A singlet exciton has higher energy than a triplet exciton because of an exchange interaction. Therefore, low energy can be upconverted to a high energy exciton state by triplet excitation annihilation
vol.43
- Cover
- Energy Technology for Carbon Neutrality
- Mehr Licht!: Realizing Highly Efficient Light Energy Conversion Using Organic Materials
- Decoding Life Phenomena and Intractable Diseases Through Experimental Biology and Data Science
- For a Better Interaction Between Humans and Robots
- GSFS FRONTRUNNERS: Interview with an entrepreneur
- Voices from International Students
- ON CAMPUS x OFF CAMPUS
- EVENT & TOPICS
- INFORMATION
- Relay Essay
